
Maged T Elghannam 1*, Moataz H Hassanien 1, Yosry A Ameen 1, Gamal M ELattar 1, Ahmed A ELRay 1, Mohammed D ELtalkawy, 1, Ahmed Y Montasser2
1Hepatogastroenterology Department; Theodor Bilharz Research Institute, Giza, Egypt
2Pathology Department; Theodor Bilharz Research Institute, Giza, Egypt
*Corresponding author: Maged T Elghannam, Hepatogastroenterology Department; Theodor Bilharz Research Institute, Giza, Egypt.
Received date: June 21, 2022
Accepted date: August 05, 2022
published date: September 27, 2022
Citation: Maged T Elghannam, Moataz H Hassanien, Yosry A Ameen, Gamal M ELattar and Ahmed Y Montasser. (2022) “Multiple Hepatic and Osseous Focal Lesions without Splenomegaly and/or Lymph Nodes Enlargement”, J of Gastroenterology and Hepatology Research, 3(3); DOI: http;//doi.org/09.2022/2.10134
Copyright: © 2022 Maged T Elghannam. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Background: Hepatic involvement is a common extranodal manifestation of common and some rare hematologic malignancies. Although the imaging features of more common hepatic diseases such as hepatocellular carcinoma, metastases, and infection may overlap with those of hepatic hematologic malignancies, combining the imaging features with clinical manifestations and laboratory findings can facilitate correct diagnosis. Imaging has an important role in diagnosis of hepatic focal lesions.
Case presentation: A case presented with isolated multiple hepatic focal lesions without nodal or spleen enlargement diagnosed only by immunohistochemical study and turned out to be Primary Hepatic Lymphoma (PHL). PHL is rare with roughly 100 described cases and accounts for less than 1% of all non-Hodgkin lymphomas. Osseous involvement add more challenge for the diagnosis.
Conclusion: Hepatologists must be aware of PHL as it may confuse with more common hepatic diseases mainly multifocal HCC and/or hepatic metastasis.
Background:
Multiple hepatic focal lesions can be caused by Hepatocellular carcinoma (HCC), metastasis, hematologic malignancy whether primary or secondary and infection or abscess in patients taking chemotherapy [1]. For hepatologists, it is very rare to think out of such diagnoses. The aim of reporting such cases is to shed light and add more concern on diagnostic facilities and how to proceed to diagnose with stress on the role of imaging techniques and immunohistochemical study. Ap¬propriate diagnosis and consequently management because surgery or liver-directed therapy (for HCC and metastasis) and antibiotic administration and drainage (for infec¬tion) may be obviated versus chemotherapy for hematologic malignancies. Here, we report a case of multiple hepatic and osseous focal lesions.
Case presentation:
Egyptian Male patient 52years old. He is shisha smoker until year 2006. He is not drug addict or received any hormonal therapy before.
His past history was significant only for tonsillectomy since 2006 and disc removal; plate and screw since 2014 after which he became user for NSAIDs. Medically, he is known to have NIDDM on metformin XR 1gram BID and empagliflozin 25mg tablet OD. Also, he is hypertensive on co-tareg 160/12.5mg tablet. Recently, there is history of COVID-19 affection during the last month.
The present history started 2 months ago when he feels bilateral back pains; he sought medical advice and his doctor asked for abdominal ultrasonography (Figure 1). Surprisingly, he discovered multiple variable sized hepatic complex cystic focal lesions; the largest at RT lobe measures 3.1x3.2cm and at Lt lobe measures 2.3x2.1cm on top of markedly enlarged diffuse fatty liver. His doctor asked for blood tests and CT abdomen. The laboratory tests were as follow: CBC: HB 13.6g/dL, total leucocytic count 10.2x103/mm3, platelet count 251x103 , CRP 199.4mg/L, Prothrombin time 11.7/10.6, concentration 86%, INR 1.07. APTT 29.2, total bilirubin 0.85mg/dl, direct bilirubin 0.22mg/dl, ALT 32U/L, AST 0.63U/l, serum albumin 4.7g/dl, serum creatinine 0.67mg/dl, HBsAg and Anti-HCV antibody were negative. Alfa feto protein 2.5ng/dl, CEA 1.6ng/dl, CA19-9 2U/ml, LDH 222U/L, FBS 152mg/dl. 2hpp 153mg/dl.
Triphasic CT showed multiple hypodense hepatic focal lesions at both hepatic lobes; largest one 34x32mm within segment VI with mild enhancement on post contrast series suggestive of neoplastic lesions on top of markedly enlarged fatty liver.
CT guided biopsy ordered and revealed florid inflammatory reaction. For confirmation, a second guided biopsy had been done by another doctor and revealed the same histologic diagnosis.
Whole body PET-CT examination ordered and showed variable sized metabolically active bilobar focal hepatic lesions, the largest and most active is seen at the RT lobe segment VI measuring 5.5cm in diameter. In addition, there are 2 metabolically active marrow based osseous lesions at L1 vertebral body and proximal RT femoral shaft. The suggested provisional diagnosis includes lymphomas vs metastatic multifocal primary hepatic malignancy (Figure 2).
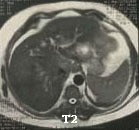
The patient referred to consultant hepatologist. Physical examination was impressive only for hepatomegaly. He asked for dynamic MRI and immunohistochemical staining of the tissue biopsy. The dynamic MRI showed multiple hepatic bilobar mass lesions the largest involving segment VI measuring 50x50mm in cross sectional diameters. The lesions displaying heterogeneous low T1 and higher T2 signal with some element of diffusion restriction along its periphery. The contrast uptake is seen in the anterior phase with washout thereafter. There is lower lumber transpedicular fixation. He considered metastatic vs multifocal HCC (Figure 3).
The immunohistochemical sections were treated against CK-PanCK, CD10, Bcl6, Mum 1, and Ki 67. The results showed large neoplastic lymphoid cells which are strongly positive for CD 20 and are positive for BCL6 and Mum1. The findings are consistent with large B cell lymphoma, post germinal center origin (Activated B cell type) (Figure 4). The patient referred to oncologist to start chemotherapy.
Discussion:
Malignant lymphomas; Hodgkin disease (HD) and non-Hodgkin lymphoma (NHL), account for approximately 5-6% of all malignancies [2]. Primary hepatic lymphoma (PHL) is defined as lymphoma that is confined to the liver and perihepatic nodal sites at patient presentation, without distant involvement [3]. PHL is rare with roughly 100 described cases and accounts for less than 1% of all non-Hodgkin lymphomas [1]. The incidence of PHL has increased in recent years, particularly in patients with human immunodeficiency virus (HIV) infection, predominantly because of im¬munosuppression. Pathologically most cases of PHL are of B-cell lineage [4].
PHL cell infiltration of the liver with hepatomegaly is more common in NHL than in HD, with 16-43% of cases showing hepatic involvement [5]. More than 50% of patients with PHL present with right upper quadrant pain or jaundice. The B symptoms (systemic symptoms) of lymphoma, such as fever and weight loss, are found in about one-third of patients with PHL [3]. PHL is com¬monly associated with viral hepatitis B and C and Epstein-Barr virus, but the pathophysiology of PHL is poorly understood [6].
Imaging has an important role in diagnosis of hepatic focal lesions. Lymphomatous involvement of the liver may manifest at imaging as a discrete focal liver mass or masses, diffuse infiltrating disease, or an ill-defined mass in the porta hepatis. The most common imaging manifestation of PHL is a solitary discrete lesion, which is seen in about 60% of cases. Multiple lesions are seen in 35%–40% of patients [7], although one lesion is likely to be dominant. Diffuse infiltration is uncommon in PHL and indicates a poor prognosis [4]. At US, the nodules usually are hypoechoic or, rarely, anechoic and may resemble cysts. The absence of posterior acoustic enhance¬ment indicates that the lesions are solid. At CT, lymphomatous nodules commonly have soft-tissue attenuation but enhance to a lesser de¬gree than the liver parenchyma on arterial, portal venous, and delayed phase images. The lesions may demonstrate hemorrhage, necrosis, or a rim-enhancement pattern [7]. Calcification is rare in the absence of treatment. A multiphase CT study is not indicated for diagnosis of hepatic lymphoma because the lesions typically are hypo¬vascular in all phases. At MR imaging, the nodules tend to be hypo-or isointense on T1-weighted images and moder¬ately hyperintense on T2-weighted images, with an enhancement pattern similar to that seen at CT. At T2-weighted MR imaging, a “target” appearance, with a hyperintense poorly enhancing center and peripheral enhancement, has been de¬scribed in about 15% of lesions. FDG PET/CT typically demonstrates avid hyper-metabolism and is usu¬ally the imaging modality of choice for staging and for assessing treatment response [8]. Hepatologist must be aware of PHL as it may confuse with more common hepatic diseases.
Imaging findings of lymphadenopa¬thy below the level of the renal veins, poor lesion enhancement in all contrast-enhanced phases, and vascular encasement without thrombosis favor a diagnosis of lymphoma. Imaging findings of arterial phase enhancement, delayed contrast material washout with capsular enhancement, and vascular thrombosis suggest HCC. In general, hepatic lymphomas are avidly hypermetabolic at PET, while most HCCs are not [9,10,11,12]
PHL is primarily treated with chemotherapy. R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) is the most common chemotherapy regimen for advanced diffuse large B-cell lymphoma (DLBCL). This regimen is generally given every three weeks for 6 to 8 cycles.
Figures’ Legands
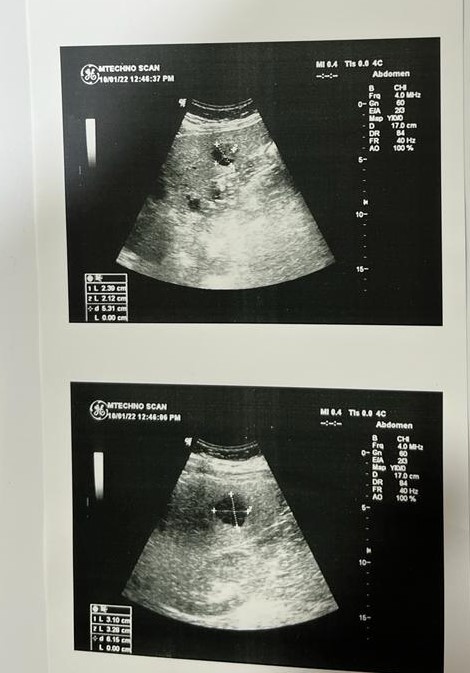
Figure 1: Abdominal ultrasonography: Multiple variable sized hepatic complex cystic focal
Lesions.

Figure 2: Whole body PET-CT: Variable sized metabolically active bilobar focal hepatic lesions in addition to active marrow based osseous lesions at L1 vertebral body and proximal RT femoral shaft.

a

b
c

d
Figure 3: Abdominal MRI: Heterogeneous low T1 and higher T2 signal with some element of diffusion restriction along its periphery.
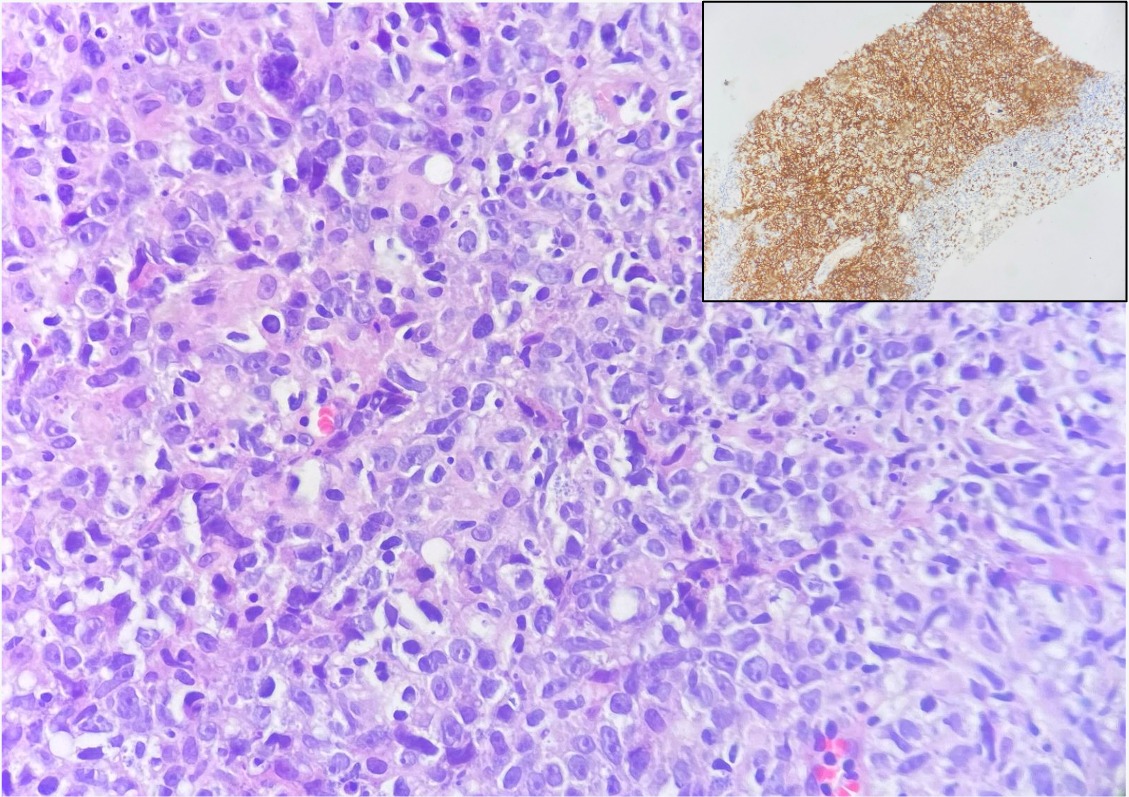
Figure 4: Histopathology and Immunohistochemical staining: Diffuse sheets of large lymphoid cells with large irregular nuclei, focally showing prominent nucleoli and many nuclear debris (H&E x400), diffusely positive for CD20 (inset, immunostain x100).
Abbreviations:
Primary Hepatic Lymphoma: PHL
Hepatocellular carcinoma: HCC
Nonsteroidal anti-inflammatory drugs: NSAIDs
C Reactive Protein: CRP
Alanine Transaminase: ALT
Aspartate Transaminase: AST
Carcinoembryonic antigen: CEA
Fasting blood sugar: FBS
Human immunodeficiency virus: HIV
Ultrasonography: US
Magnetic Resonance: MR
Computerized Tomography” CT
Positron Emission Tomography and Computed Tomography: PET-CT